MEDICAL FACE SHIELDS
VERSION A
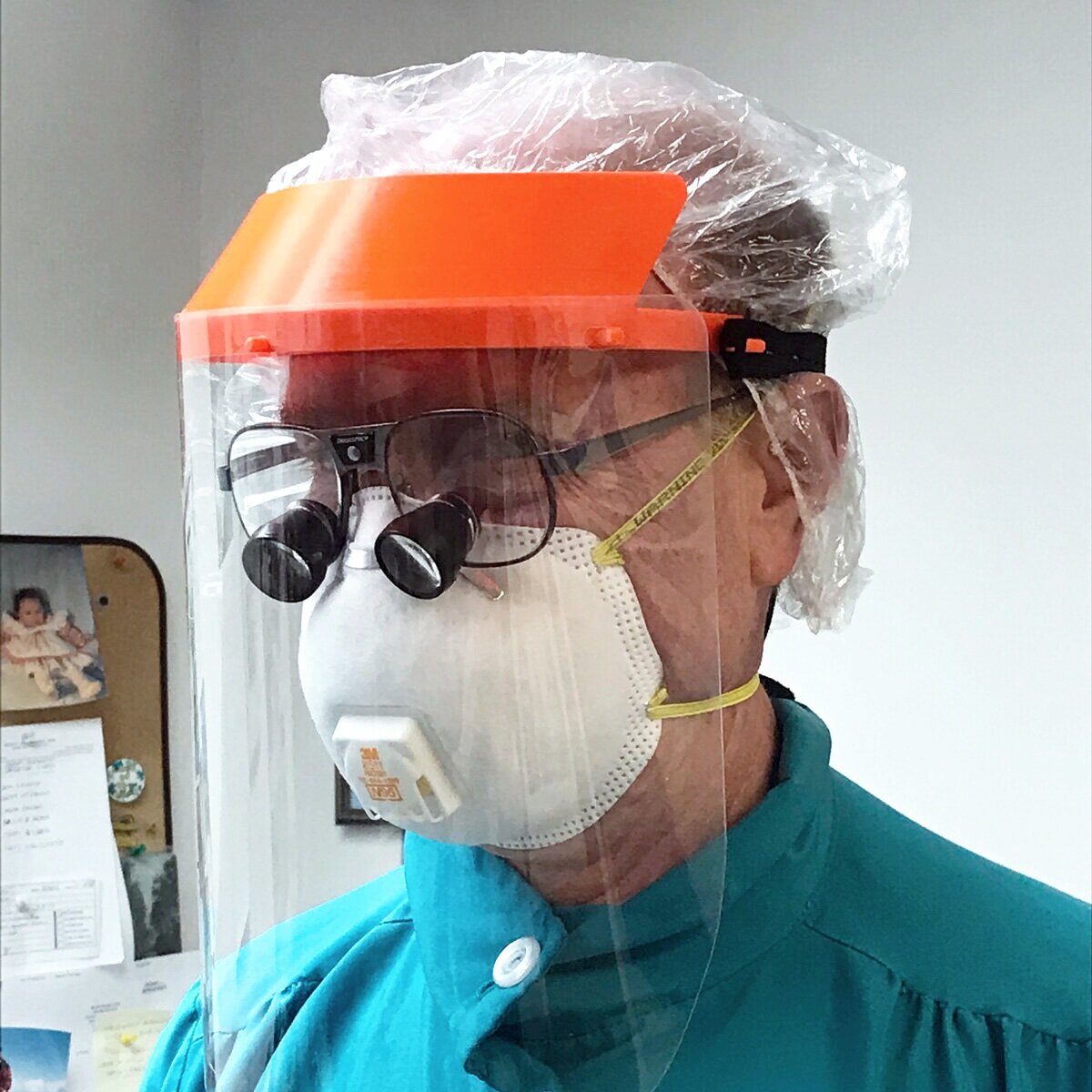
DESCRIPTION: Efficient face shield design that can be produced quickly. The National Institute of Health (NIH) has reviewed and approved this design in a clinical setting, visit their design review for more info.
quick and easy to manufacture
headband or transparent visor can be replaced when worn out or broken
comfortable to wear, does not require additional elastic band
DESIGN: 3D Verkstan
FABRICATION TIME: 45 minutes
COST OF MATERIALS: $2
VERSION B
DESCRIPTION: Adaption from the PRUSA design, includes an extra protective shield on the headband to reduce exposure to aerosol. The National Institute of Health (NIH) has reviewed and approved this design in a clinical setting, visit their design review for more info.
limits aerosol exposure, while providing top ventilation
study design, re-usable, transparent visor can be replaced when worn out
comfortable to wear + easy to take on and off
DESIGN: DTM V3.1
FABRICATION TIME: 120 minutes
COST OF MATERIALS: $5
EAR SAVERS
VERSION A
DESCRIPTION: 3D printed surgical mask strap for relieving tension for extended use.
surgical mask tension release band for ear comfort & extended use
to be worn with a N95, surgical or face mask
quicky and easy to manufacture
DESIGN: Ken Lord
FABRICATION TIME: 15 minutes
MATERIALS: PLA
COST OF MATERIALS: $0.20
VERSION B
DESCRIPTION: An alternative design for a surgical mask strap that can be cut on a laser machine.
surgical mask tension release band for ear comfort & extended use
to be worn with a N95, surgical or face mask
quicky and easy to manufacture
DESIGN: Glowforge
FABRICATION TIME: 20 seconds
MATERIALS: PETG
COST OF MATERIALS: $0.05
Safety INFORMATION
The Haystack Fab Lab is following guidance from the NIH, FDA, and other health experts on producing PPE that is safe for use in a clinical setting. All parts produced in the lab are disinfected (sterilized) with isopropyl alcohol (99%). We also have setup a “touch-free” pick up system to get PPE to people as safely as possible.
From the National Institute of Health’s COVID response page:
The FDA has authorized production of protective face shields outside of the normal clearance pathway during the COVID-19 pandemic, based on Part 5, section D of the “Enforcement Policy for Face Masks and Respirators During the Coronavirus Disease (COVID-19) Public Health Emergency Guidance for Industry and Food and Drug Administration Staff." This face shield has undergone review in a clinical setting and is recommended when fabricated as instructed.